Lithium-ion batteries have a wide range of industrial applications. But what is behind the lithium battery technology and what are the challenges involved in the raw materials used? GS Yuasa gives insight.
Lithium batteries consist of several raw materials, and lithium is not the largest component of them. (Photo: Matthew Gilbert @ AdobeStock)
The triumph of lithium (Li) cells has been unstoppable since the early 1990s. While Li (LiB) batteries were initially reserved for portable devices such as phones or laptops, they can now be found in a variety of private and industrial applications. development also Batteries have a history of development. In this way, better technology has always replaced its predecessor without completely replacing it as a rule. There were technical as well as economic reasons for that. For example, the nickel-metal hydride (NiMH) battery has replaced the nickel-cadmium (NiCd) technology and the lithium-ion (LiB) battery has replaced the existing NiMH battery and lead cell.
Lithium battery – what’s behind the technology?
The demand for lithium battery today is more than ever: Various applications from the industrial, consumer and automotive sectors depend on energy storage. Today’s Li batteries can replace various other batteries such as lead cells, but this will not always make sense from an economic and technological point of view. However, there are also different types of lithium batteries today, which differ mainly in their anode and cathode groups and therefore in their properties. Depending on the application, they offer both advantages and disadvantages.
Li batteries are now being replaced because energy density, cycle behavior and charging performance are constantly being improved. However, all the technologies are still in place and still reasonably used in many applications. From a technical point of view, LiB could replace all other variants today, but this would not make sense either in terms of requirements or in terms of costs. For the future, this means that the coexistence of different battery technologies will last for a very long time, but the importance of Li batteries will increase.
What advantages do lithium batteries offer?
The advantages of LiB include much better cycle behaviour, better charge/discharge performance, higher energy density, very low self-discharge, and an extremely wide temperature range in which the battery can operate. Unlike a lead (Pb) cell, a Li cell does not require venting and, in addition to saving weight, requires significantly less space.

E-mobility: battery and safety
(Photo: AdobeStock_277540900)
How are electric car batteries made better and are they safe? Proven and new battery technologies from development to recycling, fire protection from simulation to materials to battery management and safety concepts, as well as testing procedures from EMC to safety. The technologies behind it can be found here.
What types of Li cells are there?
But how famous is the lithium cell really? Even the label can be misleading. With the types just mentioned, as with all other battery technologies, the name usually results from the cathode material used. In this regard, the general term Li battery can only be used as a file Understand the collective term because it covers a large number of cathode and anode combinations that provide LiB with different properties.
Lithium contains the smallest share of the battery. According to a 2019 ADAC publication, a 400 kg motor battery contains 33 kg of graphite, 12 kg of nickel, 12 kg of cobalt, 11 kg of manganese and only 4 kg of lithium. It is therefore important to look at the relevant cathode and anode combination in order to clearly identify the battery and determine its characteristics and suitability. GS Yuasa works here with four groups of cathode and anode:

Lithium cobalt oxide 3.7 V/Z (LiCoO2, LCO for short)
This mixture has the highest energy density, but is also correspondingly expensive due to the limited availability of raw materials. According to the USGS from 2019, the global deposit here is only about 7 million tons. So GS Yuasa uses LiB technology with this technology in space travel or in underwater applications, where weight and space play a very important role.

Parent topic: e-mobility
(Photo: Adobe Stock, Hüthig)
In this “Electronic Mobility” main topic, it’s all about the technologies used in electric and hybrid vehicles and charging stations: from semiconductors to power electronics to electronic hubs, from batteries to safety to materials and lightweight construction as well as testing and infrastructure. Learn more here.
Lithium manganese oxide 3.7 V/Z (LiMn2O4, LMO for short)
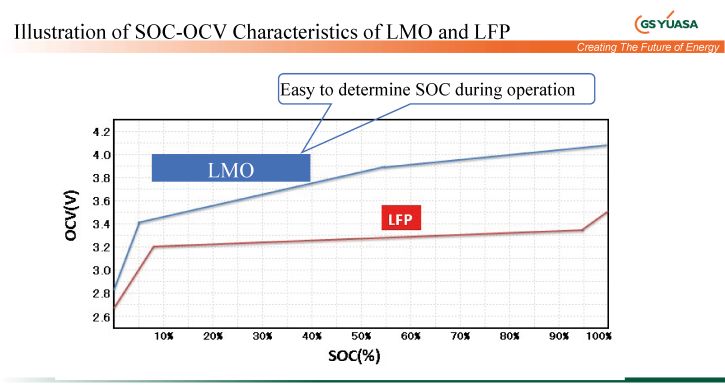
Although the cell has a lower energy density than LCO, it also brings with it lower costs and very good charge/discharge performance. Due to the linear SOC voltage characteristic, it is also possible to define a good SOC (state of charge) here.
This LiB technology is suitable for a wide range of industrial applications, in particular for general energy storage, peak load balancing, and uninterruptible power supply. According to 2019 USGS, there is a global deposit of 810 million tons of this raw material. The cell used and preferred by GS Yuasa for industrial purposes has a nominal capacity of 50 Ah and can be charged up to 4 CA to be discharged. It reaches a 75 percent charge level in 20 minutes and delivers a full 95 percent of nominal capacity at -20°C. Charged with up to 2.5 CA and the cell achieves 11,000 cycles at 70% EOL (end of life) and 25°C.

Lithium, Nickel-Manganese Cobalt 3.7 V/Z (LiaNixMnyCozO2, NMC for short)
This combination promises high energy density at affordable costs and is currently used as a classic motor battery. With 89 million tons of deposits worldwide (according to USGS 2019), nickel is a fairly rare material, but it is essential in the context of growing electrical mobility.
Lithium iron phosphate 3.2 V/Z (LiFePO4, short LFP)
Currently the most prevalent form of LiB has a wide range of applications in the consumer and industrial sectors. Unlike the LMO type, the LFP battery has a very flat discharge curve, which makes it very difficult to determine the SOC (state of charge), i.e. residual capacity. According to USGS 2019, lithium itself has a global deposit of 17 million tons. Here, too, the value means a bottleneck with regard to electrification of traffic or the implementation of electric mobility.
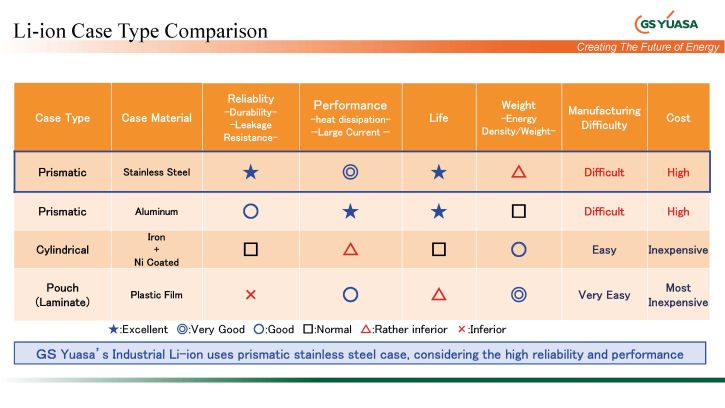
The mixture of raw materials not only plays an important role in Li battery, but also plays the final shape of the cell. Different designs have advantages and disadvantages, which are shown in the following overview.

Availability of raw materials and recycling
These explanations illustrate the scarcity and sometimes monopoly of raw materials, which an efficient recycling process requires. For example, if transportation electrification is to be successful, this requires the use of raw materials and efficient recycling. Before recycling, the “second life” of the battery, that is, using it for the second time, begins with the reuse of used batteries from electric cars in stationary energy storage systems. The capacity of the drive batteries is still 70 to 80 percent when they are no longer suitable for use in a vehicle. However, at this point, LiB is still in a state of SOH (state of health), which prohibits it from being sent to the recycling process. So it makes sense to use it as a stable storage and significantly extend its service life.
Subsequent recycling is already possible in principle, but there are not yet enough batteries to run this process economically on an industrial scale.
a company

“Certified tv guru. Reader. Professional writer. Avid introvert. Extreme pop culture buff.”








More Stories
Samsung Quantum Dot TV: Art meets technology
Pitch: €56m for energy startup Reverion
Plastoplan: Plastics for Energy Transition