March 19, 2024
Ricken
– Discovery of a membranous compartment in yeast cells that serves as a scaffold for the formation of the Golgi apparatus –
Takuro Tojima, senior scientist, Yasuyuki Suda, visiting scientist, researcher Nako Kana, full-time researcher Kazuo Kurokawa, and Akihiko Nakano, deputy team leader, Living Cell Ultra-Resolution Imaging Research Team, RIKEN Photonics Research Center, Living Cell Ultra-Resolution Imaging Research Team (Center Photonics research in living cells, the research team led by the Deputy Director of the Center)Golgi body[1]We have revealed the detailed spatio-temporal dynamics of the human body from its birth to its disappearance.
It is expected that the results of this research will contribute to clarifying the mechanisms of various diseases and viral infections resulting from disruption of the mechanisms of protein transport and sorting within cells.
Eukaryotic cells, including humans and yeast, contain various membrane structures called organelles. One of them, is the Golgi bodysmall cell body (ER)[2]a various collection ofCargo protein[3]Take inSugar lock adjustment[4]It plays a central role in transporting substances within cells, sorting and transporting each substance to where it should work.
This time, the research team has developed its own featureHigh-speed confocal microscopy (SCLIM) system[5]Using this method, we precisely observed the spatiotemporal dynamics of the Golgi apparatus in living yeast cells. As a result, for the first time in yeast cells, AER-Golgi intermediate compartment (ERGIC)[6]We have discovered a membranous compartment called the Golgi apparatus, and we have found that the Golgi apparatus is born when the properties of this membrane gradually change (cisternal maturation). Subsequently, the Golgi apparatus as wellTrans-Golgi network (TGN)[7]We also demonstrate the detailed ripening process in dedicated sorting and transport compartments.
This research was conducted in the scientific journaleLife“It will be published in the online version (on March 19, 5:00 PM JST).
background
All living things are made up of cells, which serve as the basic unit of life. The surface of cells is covered by a membrane (cell membrane) made of lipids. Within the cell there are different types of membrane-bound organelles, each of which plays a unique role in maintaining the life activities of the cell.
The Golgi apparatus, a cellular organelle, has a structure in which multiple membranous sacs called “cisternae” are stacked together, receiving cargo proteins made in the endoplasmic reticulum and processing them for modification such as the addition of sugar chains. To carry out the work. The Golgi apparatus contains polar cisternae, called cis cisternae, mesenchymal cisternae, and trans cisternae, depending on which side receives the cargo proteins. Modified cargo proteins are transported by the Golgi apparatus from the transient cisternae to the trans-Golgi network (TGN), where they are sorted and exported to their respective destinations.
How cargo proteins are transported from the endoplasmic reticulum to the Golgi apparatus, and how they pass between the multiple cisternae of the Golgi apparatus, have long been shrouded in mystery. Until now, deputy team leader Nakano and his colleagues have used SCLIM, which they developed independently, to alter the properties of the cis-cisternal cisternae of the Golgi apparatus. The Golgi gradually differentiates into intermediate and transient cisternae (cisternae) while retaining their cargo proteins. It has been detected.Notes 1 to 5).
However, it has remained a mystery as to where and how the cis-cisternal cisterna, which is the starting point for Golgi cisterna maturation, is born.
Research methods and results
In living yeast cells, the research team identified 20 proteins (Emp46, Ypt1, Rer1, Erd2, Grh1, Sed5, Mnn9, Mnn2, Vrg4, Sec21, Gnt1, Sys1, Imh1, Gea1, Gea2, Sec7, Tlg2, Apl6, Gga1,) Ypt32) were identified with fluorescent proteins and their detailed spatiotemporal dynamics were observed by SCLIM.
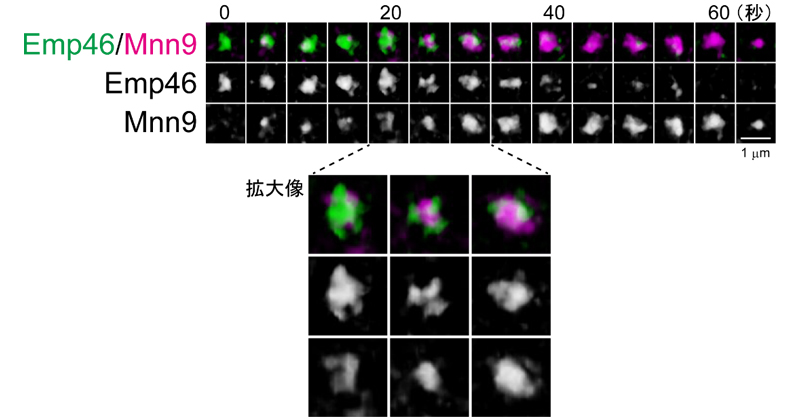
Among these proteins, Emp46 and Ypt1 are known to be molecules involved in transport between the endoplasmic reticulum and the Golgi apparatus, and these mammalian homologues are found in the ER-Golgi intermediate compartment (ERGIC), which is only found in mammalian cells. Localized in When Emp46 and Ypt1 were fluorescently labeled and observed in yeast cells, they formed compartments of similar number and size to the Golgi apparatus, and moved within the cell. These gradually transform into cis-Golgi cisternae over time (Figure 1). In other words, a compartment homologous to ERGIC in mammalian cells was first discovered in yeast cells, and it has also been revealed that the Golgi apparatus is generated when ERGIC matures. Until now, it has not been known in other species that the Golgi apparatus is born as a result of ERGIC maturation. Moreover, as a result of observing the interior of cisternae during maturation using SCLIM, proteins from earlier and later stages coexisted in single cisternae in a compartmentalized state without mixing with each other.
Figure 1: The process of cisterna maturation from the ERGIC to the Golgi apparatus
SCLIM was used to monitor the dynamics of the ERGIC-localized protein Emp46 (green) and the cis-cisternal Golgi protein Mnn9 (purple) simultaneously. A state of “cisternal maturation” in which Emp46 is gradually replaced by Mnn9 is captured. During the ripening process (enlarged image), the two are split without being completely mixed.
The research team previously revealed that the TGN, the outer part of the Golgi apparatus, is created as the Golgi apparatus matures.Note 5). This time, we also observed the transport process in detail, and found that the adapter protein AP-3 (Apl6), previously thought to be located in the TGN, to export cargo proteins to the vacuole, is not actually transported to the TGN but to the Golgi apparatus. We also found that Imh1, which acts as a scaffold to receive cargo proteins at the TGN, mainly expressed in the Golgi cisternae (Figure 2).
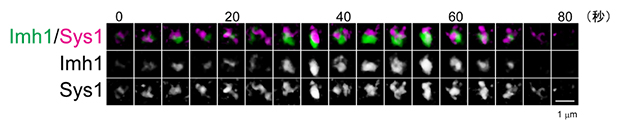
Figure 2: Spatiotemporal dynamics of Golgi cisternae
The dynamics of Imh1 (green) and Sys1 (magenta) were observed simultaneously by SCLIM. Sys1 is a marker for Golgi cisternae. Imh1 has been revealed to be expressed in Golgi cisternae.
In combination with previous reports, a spatiotemporal map of a total of 25 types of diverse functional proteins entering and exiting the Golgi apparatus was completed, and the cascade of processes by which the Golgi apparatus generates from the ERGIC and transforms into the TGN was elucidated (Figure 3).
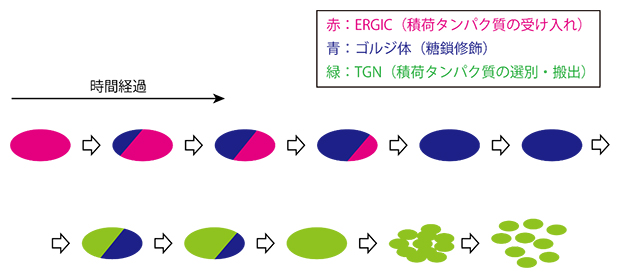
Figure 3: Spatiotemporal dynamics of Golgi apparatus formation and disappearance
This study revealed that the Golgi apparatus (blue) is formed through cisternae maturation, with the ERGIC (red) gradually changing its properties over time. The Golgi apparatus matures further into the TGN, and eventually a large number of vesicles disperse into the cytoplasm and disappear. During the transition period from the ERGIC apparatus to the Golgi apparatus and from the Golgi apparatus to the TGN, resident proteins of earlier and later stages coexisted in single cisternae in a compartmentalized state without mixing with each other.
Future expectations
From previous research, it is known that the structure and intracellular arrangement of the Golgi apparatus varies greatly in different biological species such as mammalian cells, plant cells, and yeast cells. The research team previously discovered a compartment in plant cells that has a function equivalent to that of ERGIC in mammals, and named it GECCO. (Essential component of entry into the Golgi apparatus).Note 6). The discovery of fragments homologous to those found in yeast cells strongly suggests that the molecular mechanisms of membrane traffic have been highly conserved during biological evolution.
The high-resolution, high-speed imaging technology used by the research team continues to evolve. In the future, it is expected that the dynamics of not only Golgi-localized proteins but also cargo proteins will be visualized with finer spatio-temporal resolution.
Improper transport of cargo proteins is known to be the cause of many diseases. It has recently been revealed that virus particles aggregate and accumulate in the ERGIC of cells infected with the novel coronavirus (SARS-CoV-2). If further research progresses in the future and reveals the complete molecular mechanism of cargo transport in the ERGIC, Golgi apparatus, and TGN, treatment and prevention strategies will be developed for diseases caused by membrane movement disorder and viral infections. It is believed that this will contribute significantly to the construction.
Supplementary explanation
- 1.Golgi body
A cellular organelle discovered by the Italian scientist Camillo Golgi in the nineteenth century, and its role is to modify proteins with sugar chains. It consists of sacs (cisternae) made of flat membranes, and in many biological species it has a lamellar structure containing many cisternae stacked one on top of the other, but in Saccharomyces cerevisiae cells, all cisternae disintegrate and spread within the cell. - 2.small cell body (ER)
A type of cell organelle consisting of interconnected tube-shaped membranes spread out like a network. The rough endoplasmic reticulum, to which ribosomes are attached, undergoes the synthesis of cargo proteins that are transported to the Golgi apparatus. - 3.Cargo protein
General term for membrane/secretory proteins that are newly synthesized in the endoplasmic reticulum and transported to sites of action (extracellular regions, cell membranes, various organelles, etc.) via membrane traffic routes. - 4.Sugar lock adjustment
A post-translational modification of proteins. Proteins secreted outside cells and proteins on the surface of cell membranes have sugar chains attached to different types of sugars, and added sugar chains play different roles in performing specific functions of proteins. The Golgi apparatus is a major site of glycosylation. - 5.High-speed confocal microscopy (SCLIM) system
A microscopic system independently developed by the research team. It is suitable for observing small objects such as organelles that move within living cells over time. It consists of a rotating disc confocal scanner, a high-speed piezo, a multi-wavelength spectrometer, a cryogenic image intensifier, and an EMCCD camera. SCLIM stands for high-resolution confocal live imaging microscopy. - 6.ER-Golgi intermediate compartment (ERGIC)
It was discovered in animal cells as a membrane compartment that mediates long-distance cargo transport from the endoplasmic reticulum to the Golgi apparatus. Later, the research team discovered that plants also contain a homologous membrane compartment, which they named GECCO (Golgi core entry compartment). However, until now, ERGIC/GECCO was thought not to exist in yeast cells. ERGIC is an abbreviation for ER-Golgi intermediate compartment. - 7.Trans-Golgi network (TGN)
A mesh-like membrane structure that sorts cargo proteins received from the endoplasmic reticulum through the Golgi apparatus and then sends them to various locations within the cell. It also captures and secretes recycled cargo proteins from cell membranes. It consists of the maturation of the external cisternae (cisterna transients) of the Golgi apparatus. TGN isvia-Abbreviation for Golgi network.
Research support
This research is supported by the Japan Society for the Promotion of Science (JSPS) Grant for Scientific Research (S), “Complete elucidation of the selective transport mechanism centered on the Golgi apparatus using ultra-resolution live imaging (Research Representative: Akihiko Nakano)” Same as Basic Research (A)” Building an integrated model of the membrane traffic mechanism centered on the Golgi apparatus (Research Representative: Akihiko Nakano)” Same as New Academic Area Research (Research Area Proposal Type) “GPI Anchor at the Emergency Room Exit Site, Analysis of Protein Sorting and Transport Regions (Principal Investigator: Akihiko Nakano ), Basic Research (C) “Elucidating the mechanism of neural circuit formation through visualization and manipulation of membrane traffic (Principal Investigator: Takuro Tojima),” Japan Science and Technology Agency (JST) Strategic Creative Research Promotion Project (JST) CREST “Glycosylation control based on elucidation Dynamics of the Golgi Apparatus (Principal Investigator: Koichi Kato)” was supported by this project. BioResources (HeLa RCB0007 cells) provided by the RIKEN BioResource Research Center were used in this study.
Original paper information
- Takuro Tojima, Yasuyuki Suda, Natsuko Jin, Kazuo Kurokawa, and Akihiko Nakano, “Spatiotemporal anatomy of the Golgi apparatus and the intermediate ER-Golgi compartment in budding yeast,” eLife, 10.7554/eLife.92900
Presenter
Ricken
Photonics Research Center Live Cell Ultra-Resolution Imaging Research Team
Senior researcher Takuro Tojima
Visiting scholar Yasuyuki Suda
Researcher Jin Natsuko
Full-time researcher Kazuo Kurokawa
Vice captain Akihiko Nakano
(Deputy Director of the Photonics Research Center)
 Takuro Tojima
Takuro Tojima
 Akihiko Nakano
Akihiko Nakano
Press attaché
Public Relations Office RIKEN Press Office
Inquiry form
Inquiry regarding industrial use

“Travel maven. Beer expert. Subtly charming alcohol fan. Internet junkie. Avid bacon scholar.”






More Stories
The Alexa-equipped smart display “Echo Show 5” is compact but has many functions | Gizmodo Japan
OPPO smartphone with Android 14 operating system. “Functional differences” occur in some models – OPPO Lab
The brightest gamma-ray burst in history turned out to be an ordinary supernova