[الشكل 1: رصد شياطين الغبار على المريخ في مارس 2012 (Credit: NASA / JPL-Caltech / UA)]
MarsIt has a thin atmosphere and weather phenomena.Particularly noticeableSandstormAnd sometimes they grow to cover the entirety of Mars.
Grains of sand swirling in a sandstorm rub against each other, generating static electricity. What happens after static electricity happens…….doesn’t cause lightning like Earth does. Because Mars’ atmosphere is thin, static electricity is discharged 100 times more easily than on Earth. The phenomenon of electrical discharge causes electrons to escape from molecules in the atmosphere, so phenomena such as the aurora borealis occur on Earth. This electrical discharge phenomenon is expected to appear as a faint light like the aurora borealis rather than a strong light like a bolt of lightning, but it has not yet been imaged by the Mars rover.
Research team such as Alien Wang of St. Louis Washington University have shown that this degassing phenomenon may have more meaning than “twilight in a sandstorm.” In the background, it is believed to be one of the five elements migrating to Mars.chlorine“there.
The five elements that move on the surface of Mars are hydrogen, carbon, oxygen, sulfur and chlorine. One of the characteristics of chlorine that distinguishes it from other elements is that it tends to form chemically stable substances called chlorides. Chlorides such as sodium chloride form layers of salt water and salt pans on Earth, and Mars also has a similar geological structure on its surface.
There are a few ways to liberate chlorine from chlorides, and one of them is electrolysis, which is also used in the industrial field. Chlorine can also be released on Mars if electrical discharges from dust storms interact with these chlorides.
Previous research has shown that perchlorates can form from chlorides during electrostatic discharges in carbon dioxide-rich atmospheres such as those on Mars. However, this is a simulation result, not an actual experiment.
In this study by Wang et al., this theory was verified through experiments. In order to reproduce the Martian atmosphere, the chamber was filled with carbon dioxide diluted without moisture, and potassium chloride and magnesium chloride were prepared in the form of chlorides. The research team collected and analyzed the solids and gases produced in the chamber after applying the electrical discharges expected in a medium-sized dust storm on Mars.
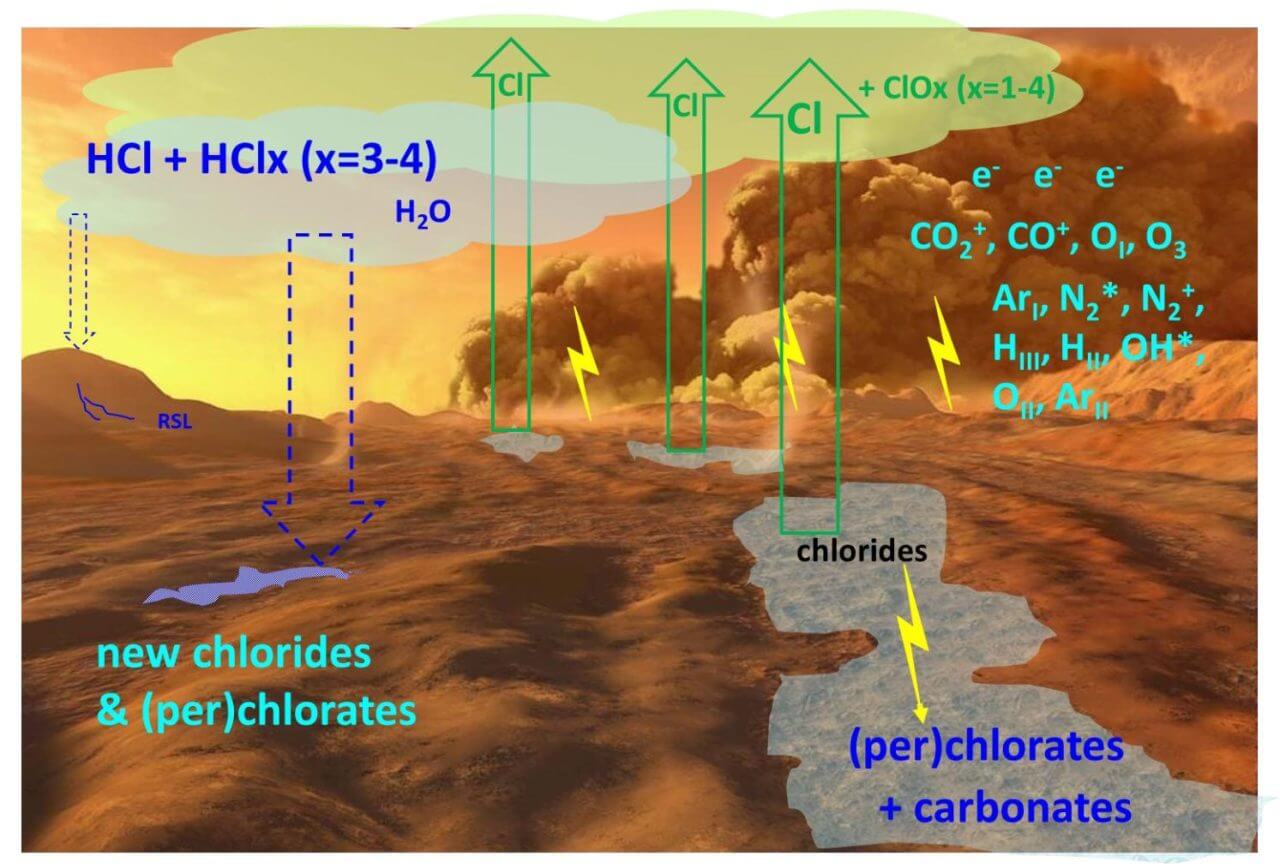
[▲ الشكل 2: تداول الكلور على سطح المريخ هو مبين في هذه التجربة. التصريفات الكهربائية التي تسببها العواصف الرملية تكسر الكلوريدات إلى ذرات كلور ، والتي يتم إطلاقها في الغلاف الجوي وتنتقل بعيدًا ، أو تصبح مصدرًا للكلورات الموجودة على سطح الأرض (Credit: Wang، et.al.)]
The result of the 7-hour unloading experiment,It has been shown that the decomposition of chloride gives rise to chlorine atoms, which are released as a gas. The ratio is about 1 chlorine atom to 100 chloride molecules.The release of chlorine from chlorides plays an important role in transporting chlorine across Mars, as chlorine atoms released into the atmosphere can easily travel long distances before falling elsewhere.。
It was also found that carbonates and perchlorates are formed, although less than chlorine atoms. Carbonates are thought to result from the reaction of metal ions from the decomposition of chlorides with carbon dioxide in the atmosphere. In addition, perchlorate is thought to result from the reaction between chlorine atoms and metal ions, as well as oxygen atoms produced as a decomposition product of carbon dioxide.
Martian soil has been shown to be unexpectedly rich in perchlorate. Perchlorate can also be produced by photochemical reactions in sunlight, but the amount of perchlorate in the soil was 10 million times higher than the amount expected from photochemical reactions, which is a big difference between the expected and actual values.There is a gap. In this experiment, the hypothesis that high concentration perchlorate was formed due to the drainage phenomenon caused by sandstorms was verified for the first time. Electrical discharges in dust storms since the late Amazonian period (3 billion years ago to the present), when the current Martian climate is thought to depend on concentrations of perchlorate and carbonate in the soil. It is assumed to have been the main driving force in the chlorine cycle.
Also, in the dust storms of 2018 and 2019, high concentrations of hydrogen chloride were found in the Martian atmosphere. Hydrogen chloride can only exist in the Martian atmosphere for a few months, which can be explained by assuming that it also originates from chlorine atoms supplied to the atmosphere by electrostatic discharge.
Estimating the transport of elements across Mars is one of the key factors for narrowing down Mars’ past climate. The demonstration of this experiment strongly suggests that dust storms may have been the main cause of chlorine migration on Mars for many years.
source
- Alian Wang et al. “Quantitative measurement of carbonates, oxychlorines, and chlorines generated by heterogeneous electrochemistry generated by Martian dust activity”. (Geophysical Research Letters)
- Talia Oglior. “Study identifies global impact of electricity in dust storms on Mars”. (Washington University in St. Louis)
- Zhongchen Wu et al. “Formation of Perchlorates on Mars Through Plasma Chemistry During Dust Events”. (Earth and Planetary Science Letters)
- Talia Oglior. “Electricity in Martian dust storms aids the formation of perchlorates”. (Washington University in St. Louis)
Text: Rare Aya

“Travel maven. Beer expert. Subtly charming alcohol fan. Internet junkie. Avid bacon scholar.”






More Stories
It's better to call it a digital camera. The Xperia 1 VI lets you take any kind of photo | Gizmodo Japan
Google may be developing a new device called “Google TV Streamer” to replace “Chromecast”
What do you want to talk about? “Persona 3 Reload” recommendation campaign is running until July 31st! |.Persona Channel