Researchers from the Massachusetts Institute of Technology (MIT) have recently succeeded, for the first time, in showing proton transfer on the surface of an electrode in detail. Their results could be the key to more efficient fuel cells and batteries.
There are still unresolved problems in the context of the energy transition. Will future batteries still rely on lithium-ion technology? How can efficient fuel cells be developed? And: Are there perhaps other alternatives in terms of power supply?
More and more researchers around the world are asking themselves these and many other questions. world From Massachusetts Institute of Technology The Massachusetts Institute of Technology (MIT) revealed the secrets of proton transport in a recently published study. This is a crucial step in many energy technologies, such as the development of fuel cell technology and electrolyzers to produce hydrogen gas.
The team recorded in detail how electron transfers coupled to protons at the electrode surface occur. These findings could help develop more efficient fuel cells, batteries, and other energy technologies.
Proton transport: improved fuel cell technology possible
The researchers developed a model of the chemical reactions of proton-coupled electron transfer using electrodes with molecularly predefined proton binding sites. The team, led by Yogesh Surendranath, a professor of chemistry and chemical engineering at MIT, focused primarily on understanding the coupling of electrons and protons at the surface site.
The scientists were able to closely monitor how changes in the pH of the electrolyte solution surrounding the electrode affected the speed of proton movement and the flow of electrons within the electrode. But there were also some challenges.
For example, electrode surfaces are usually very heterogeneous. So the MIT team developed a method to design electrode surfaces that gave them more precise control over electrode surface composition.
Different pH value leads to different reaction
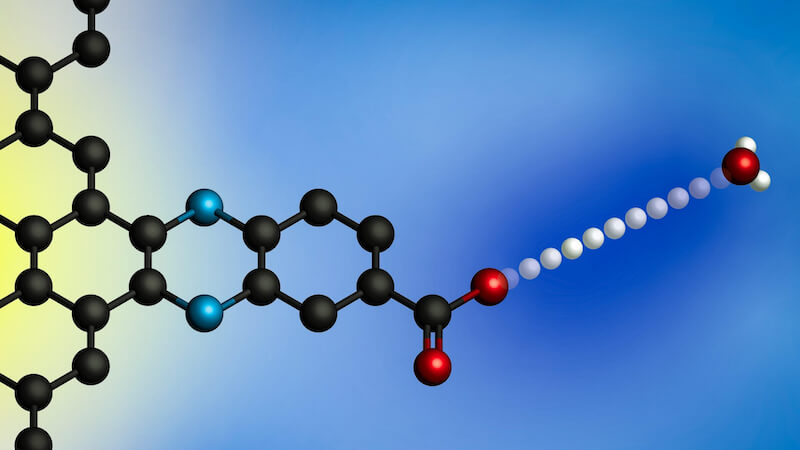
Its electrodes consist of graphene sheets with ring-shaped organic compounds on the surface. A negatively charged oxygen ion is attached to each link. This ion can absorb protons from the surrounding solution.
The results showed that the pH of the surrounding solution has a significant effect on the transfer rate. The highest rates occurred at the extreme ends of the pH scale—pH 0, which is the most acidic, and pH 14, which is the most basic. The team then developed a model based on two possible interactions that could occur at the electrode to explain these results.
This discovery could reshape the way we look at fuel cells and batteries. This would make the technologies safer and more efficient. The study results could also help achieve long-term improvements that reduce the risk of overheating and thermal runaway, representing a major advance in the development of energy storage and conversion technologies.
It is also interesting:

“Certified tv guru. Reader. Professional writer. Avid introvert. Extreme pop culture buff.”





More Stories
Samsung Quantum Dot TV: Art meets technology
Pitch: €56m for energy startup Reverion
Plastoplan: Plastics for Energy Transition