「Sodium chloride (Sodium chloride)” is one of the substances familiar to us as a spice. If sodium chloride is dissolved in water in various proportions and then the water evaporates to form crystals, sodium chloride usually appears. However, when crystals form at temperatures below 0.1 °C,Sodium chloride dihydrate (NaCl 2H2O)”, a crystal is formed that combines sodium chloride and water molecules.
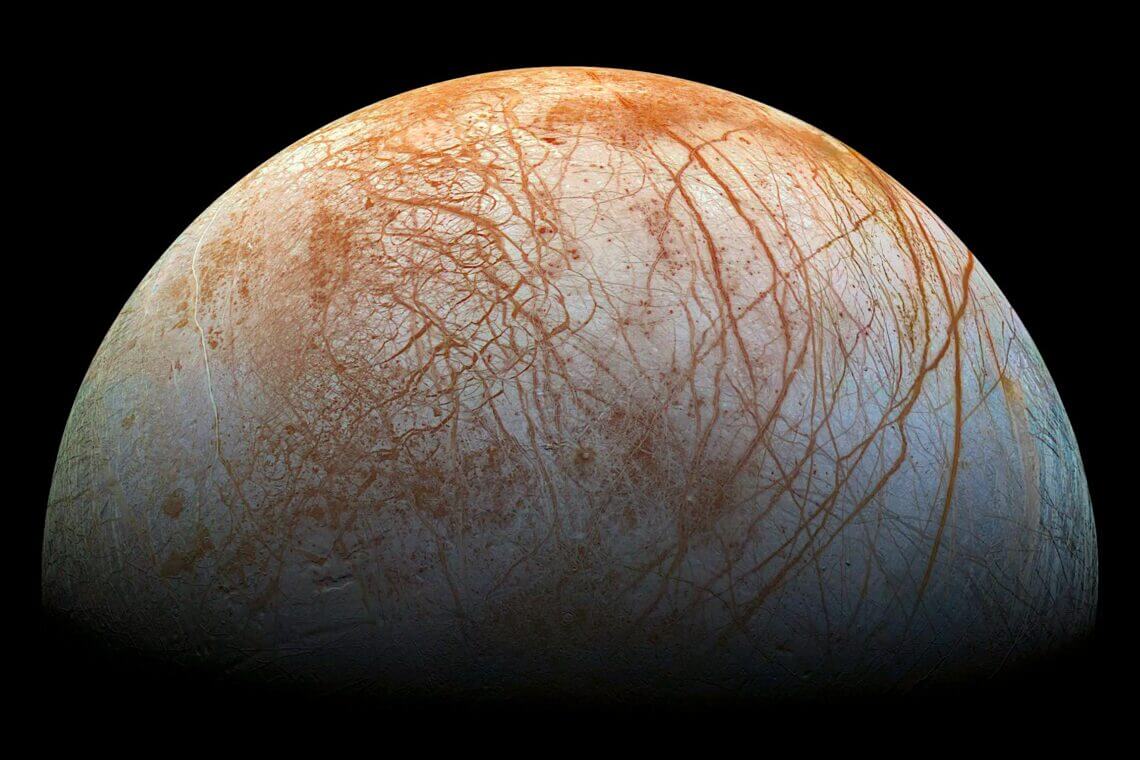
【▲ Figure 1: Europa’s surface is covered with numerous stripes that appear colorful. This is believed to originate from various materials transported from the Earth’s interior. And although the spectral data indicates the presence of sodium chloride hydrate, there is a problem that the corresponding substance could not be found[Credit: NASA/JPL/Galileo]]
Sodium chloride dihydrate decomposes into water and sodium chloride at 0.1 °C or higher, so it is not usually seen, but in cold regions, low-temperature, dry environments often generate sodium dihydrate. Since the same can be said of icy bodies such as Jupiter’s moon Europa and Saturn’s moon Enceladus, it has been estimated that there is a large amount of sodium chloride hydrate in the universe. For example, the color of the many stripes on Europa’s surface is thought to come from a variety of materials other than water brought up from the Earth’s interior. So it is not surprising that sodium chloride hydrate is present.
However, a long-standing mystery is that when we observe these icy bodies with space probes, we get different results than expected. The observational spectral data indicate the presence of an “aqueous” sodium chloride hydrate containing more than two molecules of water, but this actually indicates a different sodium chloride hydrate than the dihydrate. It remained unclear whether or not the spectral data was wrong.
A research team led by Baptiste Journeau of the University of Washington conducted an experiment in which salt water was subjected to high pressure in the laboratory. Salt water, which is water that contains sodium chloride, has a low freezing point (freezing point) in normal environments. This procedure is applied in de-icing agents, etc., but the freezing temperature also changes with pressure. The research team was originally conducting this experiment to see how the temperature of frozen brine changes under high pressure.
![[▲ الشكل 2: بلورات كلوريد الصوديوم 8.5H2O ، أحد مركبات فرط هيدرات كلوريد الصوديوم المركب هذه المرة. يتطلب التوليف ضغطًا مرتفعًا ، ولكنه يظل مستقرًا في درجات الحرارة المنخفضة حتى عندما ينخفض الضغط إلى الضغط الجوي. (رصيد الصورة: Journaux et al./PNAS)]](https://sorae.info/wp-content/uploads/2023/02/hyperhydrated-sodium-chloride-002.jpg)
[▲ الشكل 2: بلورات كلوريد الصوديوم 8.5H2O ، أحد مركبات فرط هيدرات كلوريد الصوديوم المركب هذه المرة. يتطلب التوليف ضغطًا مرتفعًا ، لكنه يظل ثابتًا عند درجات الحرارة المنخفضة حتى عند الضغط الجوي (Credit: Journaux et al./PNAS)]
but,Contrary to expectations, sodium chloride hydrate crystals formed in place of ice at pressures up to 25,000 times atmospheric pressure.This surprised the research team. As a result of examining the structure and components of the obtained crystals, the research team revealed the presence of previously unknown sodium chloride hydrate.This isA discovery that rewrites the phase diagram of a mixture of water and sodium chloride for the first time in 150 yearshad become.
![[▲ الشكل 3: التركيب البلوري لهيدرات كلوريد الصوديوم. حتى الآن ، لم يُعرف سوى نوع واحد من هيدرات كلوريد الصوديوم (يسار). من قبيل الصدفة ، تم اكتشاف نوعين جديدين من فرط هيدرات كلوريد الصوديوم هذه المرة (الوسط والأيمن). (مصدر الصورة: بابتيست جورنوكس / جامعة واشنطن)]](https://sorae.info/wp-content/uploads/2023/02/hyperhydrated-sodium-chloride-001.jpg)
[▲Fig3:CrystalstructureofsodiumchloridehydrateSofaronlyonetypeofsodiumchloridehydratehasbeenknown(left)Bychancetwonewtypesofsodiumchloridehydratehavebeendiscoveredthistime(centerandright)(Credit:BaptisteJournaux/UniversityofWashington)】[▲الشكل3:التركيبالبلوريلهيدراتكلوريدالصوديومحتىالآن،لميُعرفسوىنوعواحدمنهيدراتكلوريدالصوديوم(يسار)منقبيلالصدفة،تماكتشافنوعينجديدينمنفرطهيدراتكلوريدالصوديومهذهالمرة(فيالوسطواليمين)(Credit:BaptisteJournaux/UniversityofWashington)】
「Sodium chloride superhydrate It was also found that this crystal called “Hyperhydrated Sodium Chloride” contains two chemical components. One is”Sodium chloride 8.5H2O (Sodium Chloride 8.5 hydrate)”, the other is “Sodium chloride 13H2O (Sodium chloride-13-hydrate). Both have a greater proportion of water molecules than sodium chloride dihydrate, so they may correspond to the “hydrated” sodium chloride hydrate found in ice bodies. The high pressure within the crust of ice bodies corresponds to a high-pressure environment Created in the laboratory.This result is an unexpected finding, and it was found while the research on the conditions did not progress.By changing the experimental conditions, it is possible to find different types of supernatant sodium chloride.
Among the two sodium chloride supernatants, NaCl 8.5H2O is experimentally stable at -50 °C or less, and theoretically at -38 °C or less, even when the pressure drops to atmospheric pressure. In other words, there is a possibility that NaCl is found not only on the surface of icy bodies but also on Earth. For example, salt lakes with high salinity are known to be found within thick ice sheets in Antarctica. If sodium chloride hyperhydrates were produced at the bottom of salt lakes, there could have been some that were carried to the surface of the ice sheet.
In any case, it has not been confirmed whether the sodium chloride hyperhydrate detected this time exists in the natural world, including icy bodies. To this end, it is necessary to improve the accuracy of the data through both detailed observations by space probes and the production of larger crystals in the laboratory.
source
- The journals of Baptiste et al. “On the Determination of Hyperhydrated, Stable Sodium Chloride Hydrate under Ice Moon Conditions” (Proceedings of the National Academy of Sciences)
- Hannah Hickey. “Newly Discovered Form of Salty Ice Could Exist on Extraterrestrial Moons”. (University of Washington)
Text: Rare Aya

“Travel maven. Beer expert. Subtly charming alcohol fan. Internet junkie. Avid bacon scholar.”





More Stories
The ranking of the best survival horror games selected by the IGN US editorial team has been released! Resident Evil RE:2 ranked first
Enjoy a hot cigarette while looking at whales and tropical fish under the sea ⁉︎ “Ploom Dive” is an amazing spatial video experience using Apple Vision Pro
Apple Watch now supports sleep apnea, watchOS 11 released – Impress Watch